Can a change in the internal energy of the body occur? Internal energy
TEMPERATURE AND ITS MEASUREMENT.
[Q]=J. Q=DU.
THERMAL PROCESSES.
Melting and crystallization.
The same substance can be, under certain conditions, in solid, liquid and gaseous states, called aggregate states.
THE TRANSITION FROM SOLID TO LIQUID STATE IS CALLED MELTING. Melting occurs at a temperature called the melting point. The melting points of substances are different, because their structure is different. Melting point is a tabular value. During the melting process, the temperature does not change, because the supplied heat is spent on the destruction of the crystal lattice of the solid.
THE AMOUNT OF HEAT REQUIRED TO CONVERT 1 KG OF A SOLID AT MELTING TEMPERATURE INTO A LIQUID AT THE SAME TEMPERATURE IS CALLED SPECIFIC HEAT OF MELTING. [l]=J/kg.
CRYSTALLIZATION IS THE PROCESS OF TRANSITION OF A SUBSTANCE FROM LIQUID TO SOLID STATE. The melting point of a substance is equal to its crystallization temperature. As in the melting process, the temperature does not change during crystallization, because During crystallization, the heat that was once expended on melting the body is released. It maintains the temperature of the crystallizing body constant. In accordance with the law of conservation of energy, when calculating the amount of heat released during crystallization, the same formula is used as during melting. To show the direction of heat transfer, a minus sign is introduced into it.
Evaporation and condensation.
EVAPORATION IS THE PROCESS OF TRANSITION OF A SUBSTANCE FROM LIQUID INTO GASEOUS STATE. The molecules of a liquid attract each other, so only the fastest molecules with high kinetic energy can fly out of the liquid. If there is no heat influx, the temperature of the evaporating liquid decreases. The rate of evaporation depends on the temperature of the liquid, its surface area, the type of liquid and the presence of wind above its surface.
CONDENSATION IS THE CONVERSION OF LIQUID INTO VAPOR. In an open vessel, the rate of evaporation exceeds the rate of condensation. In a closed vessel, the rates of evaporation and condensation are equal.
When the liquid is heated, the release of air dissolved in the liquid begins at the bottom and walls of the vessel. Liquid evaporates inside these bubbles. Under the influence of Archimedean force, the bubbles break away from the walls of the vessel and float up. They enter the still unheated liquid, and the steam condenses. The bubbles collapse. At the same time, a characteristic noise is heard.
When the liquid warms up, the condensation of steam in the bubbles stops. And the vapor bubble, increasing in size due to ongoing evaporation, reaches the surface of the liquid, bursts, releasing the vapor it contains into the atmosphere. The liquid is boiling. BOILING IS VAPOR FORMATION OCCURING THROUGHOUT THE ENTIRE VOLUME OF LIQUID . Boiling occurs at a temperature called the boiling point, which depends on the type of liquid and the pressure above its surface. As the external pressure decreases, the boiling point of the liquid decreases. During the boiling process, the temperature of the liquid remains constant because the supplied energy is spent on overcoming the mutual attraction of liquid molecules.
THE AMOUNT OF HEAT REQUIRED TO CONVERT 1 KG OF LIQUID INTO VAPOR OF THE SAME TEMPERATURE IS CALLED THE SPECIFIC HEAT OF VAPOR FORMATION. [L] = J/kg. The specific heat of vaporization is different for different liquids and its numerical value is a tabular value. To calculate the amount of heat required to evaporate a liquid, the specific heat of vaporization of this liquid must be multiplied by the mass of the evaporated liquid.
When steam condenses, the same amount of heat is released that was expended on its evaporation. Intensive condensation of steam occurs at a condensation temperature equal to the boiling point.
Fuel combustion.
When fuel burns, the process of formation of carbon dioxide molecules from carbon atoms of fuel and oxygen atoms of atmospheric air occurs. This oxidation process is accompanied by the release of a large amount of heat. To characterize different types of fuel, it is introduced SPECIFIC HEAT OF COMBUSTION OF FUEL - THE AMOUNT OF HEAT RELEASED WHEN COMPLETE COMBUSTION OF 1 KG OF FUEL . [q]=J/kg. Like all other specific values, the specific heat of combustion of fuel is a tabular value. To calculate the amount of heat released during complete combustion of fuel, the specific heat of combustion of the fuel must be multiplied by the mass of the fuel.
Fuel combustion is an irreversible process, i.e. it only flows in one direction.
COULLOMB'S LAW.
A point charge is a charge located on a body, the size and shape of which can be neglected under given conditions. The law of interaction of stationary point charges was found experimentally using torsion balances by C. Coulomb in 1785.
A torsion balance is a light insulating beam with small conductive balls attached to its ends, one of which is not involved in the experiment, but only acts as a counterweight. The rocker is suspended on a thin elastic thread. A third, similarly charged ball is dropped inside through the lid of the device. One of the rocker balls is attracted to the inserted ball. In this case, the charge is divided in half between them, i.e. the balls will have charges of the same name and equal in magnitude. The balls will repel each other. The force of interaction between the balls is measured by the angle of twist of the thread. The amount of charge can be changed by removing the third ball from the device and removing the charge from it. After introducing it into the device and a new separation of charges, half of the original charge will remain on the balls. By changing the magnitude of the charges and the distances between them, Coulomb established that THE FORCE OF INTERACTION OF POINT CHARGES IS DIRECTLY PROPORTIONAL TO THE MODULES OF THE CHARGES AND INVERSE PROPORTIONAL TO THE SQUARE OF THE DISTANCE BETWEEN THEM . Point charges are those located on bodies whose size and shape can be neglected in this particular situation.
F ~ q 1 , F~q 2 , F~1/r 2 Þ F~½q 1 ½½q 2 ½/r 2 .
In addition, it was found that the force of interaction between charges in a vacuum is greater than in any dielectric medium. The quantity that shows how many times the force of interaction between charges in a vacuum is greater than in a given medium is called the dielectric constant of the medium. The dielectric constant of the medium is a tabular value.
e = F in /F. [e] = 1.
It has been established experimentally that the proportionality coefficient in Coulomb’s law k = 9 * 1O 9 Nm 2 / C 2 is the force with which two point charges of 1 C each would interact in a vacuum at a distance of 1 m.
F = k |q 1 | |q 2 |/ er 2 .
Coulomb's law is also valid for charged balls. In this case, r is understood as the distance between their centers.
OHM'S LAW FOR A CIRCUIT SECTION.
An increase in the potential difference at the ends of the conductor causes an increase in the current strength in it. Ohm experimentally proved that the current strength in a conductor is directly proportional to the potential difference across it.
When different consumers are connected to the same electrical circuit, the current strength in them is different. This means that different consumers hinder the passage of electric current through them in different ways. A PHYSICAL QUANTITY CHARACTERIZING THE ABILITY OF A CONDUCTOR TO PREVENT THE PASSAGE OF ELECTRIC CURRENT THROUGH IT IS CALLED ELECTRICAL RESISTANCE . The resistance of a given conductor is a constant value at a constant temperature. As the temperature rises, the resistance of metals increases, and that of liquids decreases. [R] = Ohm. 1 Ohm is the resistance of a conductor through which a current of 1 A flows with a potential difference of 1 V at its ends. Metal conductors are most often used. The current carriers in them are free electrons. When moving along a conductor, they interact with positive ions of the crystal lattice, giving them part of their energy and losing speed. To obtain the required resistance, use a resistance magazine. A resistance store is a set of wire spirals with known resistances that can be included in a circuit in the desired combination.
Ohm experimentally established that THE CURRENT STRENGTH IN A HOMOGENEOUS SECTION OF THE CIRCUIT IS DIRECTLY PROPORTIONAL TO THE POTENTIAL DIFFERENCE AT THE ENDS OF THIS SECTION AND INVERSE PROPORTIONAL TO THE RESISTANCE OF THIS SECTION.
A homogeneous section of a circuit is a section in which there are no current sources. This is Ohm's law for a homogeneous section of a circuit - the basis of all electrical calculations.
Including conductors of different lengths, different cross-sections, made of different materials, it was found: THE RESISTANCE OF A CONDUCTOR IS DIRECTLY PROPORTIONAL TO THE LENGTH OF THE CONDUCTOR AND INVERSE PROPORTIONAL TO ITS CROSS SECTIONAL AREA. THE RESISTANCE OF A CUBE WITH AN EDGE OF 1 METER, MADE FROM SOME SUBSTANCE, IF THE CURRENT GOES PERPENDICULAR TO ITS OPPOSITE FACES, IS CALLED THE SPECIFIC RESISTANCE OF THIS SUBSTANCE . [r] = Ohm m. A non-system unit of resistivity is often used - the resistance of a conductor with a cross-sectional area of 1 mm 2 and a length of 1 m. [r] = Ohm mm 2 /m.
The specific resistance of a substance is a tabular value. The resistance of a conductor is proportional to its resistivity.
The action of slider and step rheostats is based on the dependence of the conductor resistance on its length. The slider rheostat is a ceramic cylinder with nickel wire wound around it. The rheostat is connected to the circuit using a slider, which includes a larger or smaller winding length in the circuit. The wire is covered with a layer of scale, which insulates the turns from each other.
A) SERIES AND PARALLEL CONNECTION OF CONSUMERS.
Often several current consumers are included in an electrical circuit. This is due to the fact that it is not rational for each consumer to have their own current source. There are two ways to connect consumers: serial and parallel, and their combinations in the form of a mixed connection.
a) Serial connection of consumers.
With a series connection, consumers form a continuous chain in which consumers are connected one after another. With a series connection, there are no branches of connecting wires. For simplicity, let us consider a circuit of two series-connected consumers. An electric charge that passes through one of the consumers will also pass through the second one, because in the conductor connecting consumers there cannot be the disappearance, emergence or accumulation of charges. q=q 1 =q 2 . Dividing the resulting equation by the time the current passes through the circuit, we obtain a relationship between the current flowing throughout the entire connection and the currents flowing through its sections.
Obviously, the work to move a single positive charge throughout the compound consists of the work to move this charge across all its sections. Those. V=V 1 +V 2 (2).
The total potential difference across series-connected consumers is equal to the sum of the potential differences across consumers.
Let's divide both sides of equation (2) by the current in the circuit, we get: U/I=V 1 /I+V 2 /I. Those. The resistance of the entire series-connected section is equal to the sum of the resistances of the voltages of its components.
B) Parallel connection of consumers.
This is the most common way to enable consumers. With this connection, all consumers are connected to two points common to all consumers.
When passing through a parallel connection, the electric charge flowing through the circuit is divided into several parts, going to individual consumers. According to the law of conservation of charge q=q 1 +q 2. Dividing this equation by the charge passage time, we obtain a relationship between the total current flowing through the circuit and the currents flowing through individual consumers.
In accordance with the definition of potential difference V=V 1 =V 2 (2).
According to Ohm's law for a section of the circuit, we replace the current strengths in equation (1) with the ratio of the potential difference to the resistance. We get: V/R=V/R 1 +V/R 2. After reduction: 1/R=1/R 1 +1/R 2 ,
those. the reciprocal of the resistance of a parallel connection is equal to the sum of the reciprocals of the resistances of its individual branches.
KIRCHHOFF'S RULES.
Kirchhoff's rules are used to calculate branched electrical circuits.
The point in a circuit where three or more conductors intersect is called a node. According to the law of conservation of charge, the sum of the currents entering the node and leaving it is equal to zero. I = O. (Kirchhoff's first rule). THE ALGEBRAIC SUM OF CURRENTS PASSING THROUGH THE NODE IS EQUAL TO ZERO.
The current entering the node is considered positive, leaving the node negative. The directions of currents in sections of the circuit can be chosen arbitrarily.
From equation (2) it follows that WHEN BYPASSING ANY CLOSED LOOP, THE ALGEBRAIC SUM OF THE VOLTAGE DROP IS EQUAL TO THE ALGEBRAIC SUM OF THE EMF IN THIS CIRCUIT , - (Kirchhoff's second rule).
The direction of traversing the contour is chosen arbitrarily. The voltage in a section of the circuit is considered positive if the direction of the current in this section coincides with the direction of bypassing the circuit. The EMF is considered positive if, when going around the circuit, the source passes from the negative pole to the positive one.
If the chain contains m nodes, then m - 1 equations can be composed using the first rule. Each new equation must include at least one new element. The total number of equations compiled according to Kirchhoff’s rules must coincide with the number of sections between the nodes, i.e. with the number of currents.
PERMANENT MAGNETS.
The strengthening of the magnetic field of the solenoid when an iron core is introduced into it is due to the fact that the iron in the magnetic field is magnetized and its magnetic field, superimposed on the magnetic field of the coil, strengthens it. Iron is a highly magnetic material, which also includes nickel, cobalt, gadolinium and their compounds. The magnetization of the iron core is maintained even after it is removed from the coil. A body that retains magnetic properties is called a permanent magnet. Every permanent magnet has two poles - north and south. These are the places on the magnet where the magnetic field is greatest. Like poles of magnets repel, opposite poles attract. The field configuration of permanent magnets can be easily examined using iron filings.
Naturally magnetized pieces of iron or iron ore were already used in Ancient China for orientation on the Earth, which itself is a huge permanent magnet. The Earth's south magnetic pole is located in the area of the north geographic pole, but does not coincide with it, the north magnetic pole is in the area of the south geographic pole. The position of the magnetic poles is not constant. In addition, analysis of the Earth's sedimentary rocks suggests that the Earth's magnetic field has repeatedly changed polarity. The Earth's magnetic field plays a huge role for all life on it, because... it protects us from the stream of fast particles flying to Earth from outer space, mostly from the Sun. When this flow changes, magnetic storms are observed on Earth - short-term changes in the Earth's magnetic field, causing disruption of radio communications and deviations in the position of magnetic needles.
MAGNETIC FIELD OF CURRENT.
In 182O, Oersted discovered that a magnetic needle located next to a conductor through which an electric current flows rotates so that its axis coincides with the tangent to the circle enclosing this conductor.
In the same year, Ampere discovered the interaction of conductors with current and found the law to which this interaction obeys. The action of a current-carrying conductor on a magnetic needle and the interaction of current-carrying conductors can be explained by the fact that a current-carrying conductor creates a magnetic field in the space surrounding it, which is detected by a magnetic needle or another current-carrying conductor.
A magnetic field is a special type of matter created by moving electric charges (current) and detected by its effect on moving electric charges (current). A magnetic field propagates through space at the speed of light. It decreases with increasing distance from the current creating it. A magnetic field has energy.
To study magnetic fields, small magnetic needles are used, with the help of which a convenient way has been found to graphically represent magnetic fields using magnetic lines. A magnetic line is a line along which the axes of small magnetic needles in a magnetic field are located. The appearance of magnetic lines is easily established using small iron filings sprinkled on cardboard and introduced into a magnetic field. In this case, the sawdust, magnetized in the field, is arranged in chains along the magnetic lines. The direction of these lines is taken to be the direction that the north pole of the magnetic needle would indicate.
The magnetic lines of a straight conductor carrying current are circles, the center of which is the conductor carrying current. The direction of the lines is determined by the gimlet rule: if the translational movement of the gimlet (right screw) coincides with the direction of the current in the conductor, then the direction of the rotational movement of the gimlet handle coincides with the direction of the magnetic lines.
The magnetic lines of a current-carrying coil (solenoid) are closed curves covering the turns of the coil. The direction of these lines can be easily determined by the following rule: if you take the coil with your right hand so that the bent fingers are directed along the current in it, then the bent thumb will show the direction of the magnetic lines along the axis of the coil.
A current-carrying coil is an electromagnet similar to a permanent strip magnet. The magnetic field of a coil increases with the number of its turns and the current in it. To enhance the magnetic field, an iron core is inserted into the coil. The place where the magnetic lines leave the coil is the north pole of the electromagnet, and where they enter is the south pole.
Electromagnets are widely used in technology both for moving heavy iron parts, iron scrap, and in many electrical and radio engineering devices.
A magnetic field acts with some force on a current-carrying conductor located in it. This force is called the Ampere force and depends directly on the length of the conductor and the current strength in it. It also depends on the magnitude of the field and the location of the conductor. The direction of the Ampere force is determined by the left hand rule: if the left hand is positioned in a magnetic field so that the magnetic lines enter the palm, and four extended fingers show the direction of the current, then the bent thumb will show the direction of the force.
The effect of a magnetic field on a current-carrying conductor is used in electric motors. A DC electric motor consists of a stationary part - the stator and a moving part - the rotor. A coil is placed in the stator slots, creating a magnetic field. The rotor is a coil of many turns, to which current is supplied using sliding contacts - brushes. To increase the magnetic field, the rotor and stator are made of transformer steel sheets, insulated from each other. The rotor is driven by the Ampere force. To maintain constant rotation, the direction of the current in the rotor winding periodically changes with the help of a commutator, which in the simplest case is two half rings in contact with the brushes. As the rotor moves, the brush moves from one half-ring to another, changing the direction of the current in the rotor coil. This gives her the opportunity to turn another half turn when the current changes direction again.
Because The efficiency of electric motors (up to 98%) is much greater than that of thermal motors, so electric motors are widely used in transport, factories, etc. Electric motors are compact, do not pollute the environment, and are easy to control.
OPTICAL INSTRUMENTS.
Camera.
The camera consists of two main parts: a light-proof camera and a lens. In the simplest case, a converging lens can serve as a lens. In order for the image to be of high quality throughout the entire field of the photograph, the lenses of modern cameras are a complex system of lenses, which generally plays the role of a converging lens. The camera lens produces, on photographic film coated with a photosensitive layer, a real, inverse, and, as a rule, reduced image of the object being photographed. The camera uses a thin lens formula. To obtain a clear (sharp) image of an object, the camera lens is made movable. By moving the lens, the required image sharpness is achieved. The objects being photographed can be at different distances from the camera at the same time. Depth of field is achieved by allowing the lens window to be partially blocked by the aperture. The smaller the lens window, the clearer the objects at different distances from the camera will be in the picture.
When taking a photograph, the camera lens automatically opens for a short period of time, called exposure time. To make the image visible, the film is developed in a special solution and fixed. The resulting image is called a negative, because reverse light transmission is observed on it. Those parts of the film where more light fell are darker and vice versa. To obtain a photo card (positive), the resulting image is projected onto photo paper using a photo enlarger. The paper is then developed and cured.
Modern cameras can produce color and even three-dimensional images. Some devices immediately produce a ready-made photograph. The development of photography became cinema.
Photography is widely used for scientific purposes, technology, forensics, etc. It can make us witnesses of historical events. Art photography is widespread.
Projection apparatus.
The projection apparatus is used to obtain a real, enlarged, inverse image of bodies on the screen. If an image is obtained in transmitted light (photo and film, an image on glass), then the device is called a diascope, in reflected light - an episcope. A combination of these devices is often used - an epidiascope. A diascope consists of a light source, a condenser and an objective lens. To increase the illumination of the screen, one or more mirrors are often placed behind the light source. A condenser (two flat-convex lenses) directs the light diverging from the source into the lens. The simplest lens can be a converging lens. The object, the image of which must be obtained on the screen, is placed between the condenser and the lens. Image clarity is achieved by moving the lens.
Photo enlargers, filmoscopes, movie cameras, overhead projectors are projection devices.
Eye. Glasses.
The structure of the eye resembles a camera. It consists of: sclera - the outer part of the eye that protects the eye from mechanical damage; cornea - the anterior transparent part of the sclera; the iris with a hole of variable diameter in it - the pupil; lens - biconvex lens; vitreous humor, which fills the volume of the eye; retina - nerve endings that transmit information to the brain. The space between the cornea and the lens is filled with aqueous fluid, which mainly refracts light. The eye works on a thin lens formula. Because objects can be located at different distances from the eye, then to obtain a clear image, the curvature of the lens can change with the help of the eye muscles. The ability of the eye to give a clear image of objects located at different distances from it is called accommodation. The distance at which the eye allows one to see small details of objects without much strain is called the distance of best vision. For a healthy eye, it is 25 cm. The near limit of accommodation is about 12 cm. The depth of field is determined by the area of the pupil. The retina consists of rods, which produce black and white images, and cones, which produce color images. The image on the retina is real, reduced, inverse. Three-dimensional vision is provided by two eyes.
If the image created by the eye lies in front of the retina, then the eye is called myopic. To look at an object, a nearsighted person brings it close to his eyes and strongly strains the eye muscles. Myopia is corrected by wearing glasses with diverging lenses. The farsighted eye creates an image behind the retina. Farsightedness is corrected by wearing glasses with converging lenses. It should be noted that both myopia and farsightedness will progress if you do not use glasses, because When working, the eye muscles will become overstrained.
TEMPERATURE AND ITS MEASUREMENT.
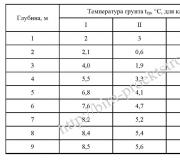
The study of thermal phenomena inevitably had to give a value characterizing the degree of heating of bodies - temperature. When bodies come into contact, as a result of the interaction of molecules, their average kinetic energy is equalized. Temperature is a measure of the average kinetic energy of molecules. It shows the direction of thermal processes, because energy is spontaneously transferred from more heated bodies to less heated ones, i.e. from bodies with higher temperatures to bodies with lower temperatures. Temperature is measured by thermometers. Temperature measurement is based on the establishment of thermal equilibrium between bodies brought into contact. In practice, the most widely used are liquid thermometers, which use a change in the volume of a liquid (mercury or alcohol) when heated. As the liquid expands, it rises through a glass tube, under which there is a scale. The reference points (i.e., the points on which the temperature scale is based) in the international practical temperature system proposed by Celsius are the melting point of ice (O 0 C) and the boiling point of water (1OOS0oTC). The distance between these points on the scale is divided into 100 equal parts. Because Since the expansion of a liquid is different in different temperature ranges, a liquid thermometer guarantees the correct measurement of only reference temperatures. Gas thermometers that use the dependence of gas volume on temperature at constant pressure or the dependence of gas pressure on temperature at constant volume have greater accuracy. Thermometers can also use the dependence of the electrical resistance of conductors and semiconductors on temperature.
INTERNAL ENERGY AND WAYS TO CHANGE IT.
Every body consists of a huge number of molecules. Molecules of bodies are constantly moving, therefore, they have kinetic energy. Molecules of solid and liquid bodies interact with each other, which means they also have potential energy. THE SUM OF KINETIC AND POTENTIAL ENERGIES OF THE MOLECULES COMPOSING THE BODY IS CALLED INTERNAL ENERGY. [U]=J. Internal energy also includes the energy of the particles that make up atoms.
The internal energy of a body can change during various thermal processes. So, when heated, for example, the speed of movement of molecules increases, and therefore their kinetic energy. When a body is heated, its volume increases, the distances between molecules change, and therefore the potential energy of their interaction also changes. The change in internal energy can be judged by the change in body temperature. As the temperature of a body increases, its internal energy increases.
Internal energy can be changed in two fundamentally different ways.
1. If work is done on a body, it heats up, i.e. his internal energy increases. If the body itself does work on external bodies, its internal energy decreases. A=DU.
2. Internal energy can also be changed by heat transfer. HEAT TRANSFER, OR HEAT EXCHANGE, IS THE PROCESS OF CHANGING INTERNAL ENERGY WITHOUT DOING WORK. Thus, a kettle standing on a hot stove receives energy through heat transfer.
There are three types of heat transfer: thermal conductivity - the transfer of energy by exchanging it between molecules during their interaction; convection - transfer of energy by flows of heated liquid or gas; radiation - the transfer of energy through electromagnetic waves. Moreover, the latter type of heat transfer does not require direct contact of bodies or the presence of any substance between them.
The measure of transferred thermal energy during heat transfer is THE AMOUNT OF HEAT IS THAT PART OF INTERNAL ENERGY THAT A BODY RECEIVES OR GIVES UP DURING HEAT TRANSFER. [Q]=J. Q=DU.
THERMAL PROCESSES.
Internal body energy cannot be a constant value. It can change in any body. If you increase the body temperature, then its internal energy will increase, because the average speed of molecular movement will increase. Thus, the kinetic energy of the molecules of the body increases. And, conversely, as the temperature decreases, the internal energy of the body decreases.
We can conclude: The internal energy of a body changes if the speed of movement of the molecules changes. Let's try to determine what method can be used to increase or decrease the speed of movement of molecules. Consider the following experiment. Let's attach a brass tube with thin walls to the stand. Fill the tube with ether and close it with a stopper. Then we tie a rope around it and begin to move the rope intensively in different directions. After a certain time, the ether will boil, and the force of the steam will push out the plug. Experience demonstrates that the internal energy of the substance (ether) has increased: after all, it has changed its temperature, at the same time boiling.
The increase in internal energy occurred due to the work done when the tube was rubbed with a rope.
As we know, heating of bodies can also occur during impacts, flexion or extension, or, more simply, during deformation. In all the examples given, the internal energy of the body increases.
Thus, the internal energy of the body can be increased by doing work on the body.
If the work is performed by the body itself, its internal energy decreases.
Let's consider another experiment.
We pump air into a glass vessel that has thick walls and is closed with a stopper through a specially made hole in it.
After some time, the cork will fly out of the vessel. At the moment when the stopper flies out of the vessel, we will be able to see the formation of fog. Consequently, its formation means that the air in the vessel has become cold. The compressed air that is in the vessel does a certain amount of work when pushing the plug out. He performs this work due to his internal energy, which is reduced. Conclusions about the decrease in internal energy can be drawn based on the cooling of the air in the vessel. Thus, The internal energy of a body can be changed by performing certain work.
However, internal energy can be changed in another way, without doing work. Let's consider an example: water in a kettle that is standing on the stove is boiling. The air, as well as other objects in the room, are heated by a central radiator. In such cases, the internal energy increases, because body temperature increases. But the work is not done. So, we conclude a change in internal energy may not occur due to the performance of specific work.
Let's look at another example.
Place a metal knitting needle in a glass of water. The kinetic energy of hot water molecules is greater than the kinetic energy of cold metal particles. The hot water molecules will transfer some of their kinetic energy to the cold metal particles. Thus, the energy of the water molecules will decrease in a certain way, while the energy of the metal particles will increase. The water temperature will drop, and the temperature of the knitting needle will slowly  will increase. In the future, the difference between the temperature of the knitting needle and the water will disappear. Due to this experience, we saw a change in the internal energy of various bodies. We conclude: The internal energy of various bodies changes due to heat transfer.
will increase. In the future, the difference between the temperature of the knitting needle and the water will disappear. Due to this experience, we saw a change in the internal energy of various bodies. We conclude: The internal energy of various bodies changes due to heat transfer.
The process of converting internal energy without performing specific work on the body or the body itself is called heat transfer.
Still have questions? Don't know how to do your homework?
To get help from a tutor -.
The first lesson is free!
blog.site, when copying material in full or in part, a link to the original source is required.
The internal energy of a body is not some kind of constant quantity: it can change for the same body. As the temperature rises body, the internal energy of the body increases as the average speed increases, and therefore the kinetic energy, of the molecules of this body. With a decrease in temperature, on the contrary, the internal energy of the body decreases. Thus, the internal energy of a body changes when the speed of movement of its molecules changes. In what ways can this speed be increased or decreased? Let's turn to experience.
On the stand (Fig. 181) there is a thin-walled brass tube, into which a little ether is poured, the tube is tightly closed with a stopper. A rope is wrapped around the tube and the rope is quickly moved in one direction or the other. After some time, the ether will boil and its steam will push out the plug. This experiment shows that the internal energy of the ether has increased: after all, it has heated up and even boiled. The increase in internal energy occurred as a result of the work done when rubbing the tube with the rope.
Bodies also heat up during impacts, extension and bending, and in general during deformation. In all these cases, due to the perfect work, the internal energy of the bodies increases.
So, internal energy bodies can be enlarged by doing work on the body. If the body itself does the work, then its internal energy decreases. This can be observed in the following experiment.
Take a thick-walled glass vessel closed with a stopper. Air containing water vapor is pumped into the vessel through a special hole. After some time, the plug pops out of the vessel (Fig. 182). The moment the cork pops out, fog appears in the vessel. Its appearance means that the air in the vessel has become colder (remember that fog also appears outside during cold weather).
The compressed air in the vessel, pushing out the plug, does work. He does this work at the expense of his internal energy, which decreases. We judge the decrease in energy by the cooling of the air in the vessel.
The internal energy of the body can be changed in another way.
It is known that a kettle of water standing on the stove, a metal spoon dipped into a glass of hot tea, a stove in which a fire is lit, the roof of a house illuminated by the sun heat up. In all cases, the temperature of bodies increases, which means their internal energy also increases. How to explain its increase?
How, for example, does a cold metal spoon dipped into hot tea heat up? First, the speed and kinetic energy of hot water molecules is greater than the speed and kinetic energy of cold metal particles. In those places where the spoon comes into contact with water, the hot water molecules transfer part of their kinetic energy to the cold metal particles. Therefore, the speed and energy of water molecules on average decreases, and the speed and energy of metal particles increases: the temperature of the water decreases, and the temperature of the spoon increases - their temperatures gradually level out. With a decrease in the kinetic energy of molecules water decreases and the internal energy of all water in the glass, and the internal energy of the spoon increases.
The process of changing internal energy, in which no work is done on a body, but energy is transferred from one particle to another, is called heat transfer. So, the internal energy of the body can be changed in two ways: performing mechanical work or heat transfer.
When the body is already heated, we cannot indicate which of the two ways this was done. Thus, holding a heated steel knitting needle in our hands, we cannot say in what way it was heated - by rubbing it or placing it in a flame.
Questions. 1. Give examples showing that the internal energy of a body increases when work is done on the body. 2. Describe an experiment showing that a body can do work due to internal energy. 3. Give examples of increasing the internal energy of a body by heat transfer. 4. Explain heat transfer based on the molecular structure of matter. 5. What are two ways to change the internal energy of the body?
Exercise.
Place a five-kopeck coin on a sheet of plywood or wooden board. Press the coin against the board and move it quickly, first in one direction, then in the other direction. Notice how many times you need to move the coin so that it becomes warm, hot. Draw a conclusion about the connection between the work done and the increase in the internal energy of the body.
Internal energy is the sum of the kinetic energies of all the particles that make up the body, and the potential energies of the interaction of these particles with each other. This includes the energy of interaction of electrons with nuclei and the energy of interaction of the constituent parts of the nucleus.
Internal energy depends on its temperature. Temperature characterizes the average kinetic energy of particles of a substance. When the temperature changes, the distance between the particles changes, therefore, the energy of interaction between them also changes.
Internal energy also changes when a substance transitions from one state of aggregation to another. Processes associated with changes in temperature or state of aggregation of a substance are called thermal. Thermal processes are accompanied by changes in the internal energy of the body.
Chemical reactions and nuclear reactions are also accompanied by a change in the internal energy of the body, because the interaction energy of particles participating in reactions changes. Internal energy changes when energy is emitted or absorbed by atoms during the transition of electrons from one shell to another.
One of ways to change internal energy is Job. So, when two bodies rub together, their temperature increases, i.e. their internal energy increases. For example, when processing metals - drilling, turning, milling.
When two bodies with different temperatures come into contact, energy is transferred from the body with a high temperature to the body with a low temperature. The process of transferring energy from one body to another, which has a lower temperature, is called heat transfer.
Thus, in nature there are two processes in which the internal energy of a body changes:
a) conversion of mechanical energy into internal energy and vice versa; at the same time work is done;
b) heat transfer; in this case, no work is done.
If you mix hot and cold water, you can verify from experience that the amount of heat given off by hot water and the amount of heat received by cold water are equal. Experience shows that if heat exchange occurs between bodies, then the internal energy of all heating bodies increases by as much as the internal energy of cooling bodies decreases. Thus, energy moves from one body to another, but the total energy of all bodies remains unchanged. This law of conservation and transformation of energy.
In all phenomena occurring in nature, energy neither appears nor disappears. It only transforms from one type to another, while its meaning remains the same.
For example, a lead bullet flying at a certain speed hits an obstacle and heats up.
Or, a piece of ice, falling from a snow cloud, melts near the ground.
Internal energy can be changed in two ways.
If work is done on a body, its internal energy increases.

If the body itself does the work, its internal energy decreases.
There are three simple (elementary) types of heat transfer:
Thermal conductivity
Convection
Convection is the phenomenon of heat transfer in liquids or gases, or granular media by flows of matter. There is a so-called natural convection, which occurs spontaneously in a substance when it is unevenly heated in a gravitational field. With such convection, the lower layers of the substance heat up, become lighter and float up, and the upper layers, on the contrary, cool, become heavier and sink down, after which the process is repeated again and again.
Thermal radiation or radiation is the transfer of energy from one body to another in the form of electromagnetic waves due to their thermal energy.
Internal energy of an ideal gas
Based on the definition of an ideal gas, it does not have a potential component of internal energy (there are no molecular interaction forces, except shock). Thus, the internal energy of an ideal gas represents only the kinetic energy of motion of its molecules. Previously (equation 2.10) it was shown that the kinetic energy of the translational motion of gas molecules is directly proportional to its absolute temperature.

Using the expression for the universal gas constant (4.6), we can determine the value of the constant α.
Thus, the kinetic energy of translational motion of one molecule of an ideal gas will be determined by the expression.

In accordance with kinetic theory, the distribution of energy across degrees of freedom is uniform. Translational motion has 3 degrees of freedom. Consequently, one degree of freedom of movement of a gas molecule will account for 1/3 of its kinetic energy. 
For two, three and polyatomic gas molecules, in addition to the degrees of freedom of translational motion, there are degrees of freedom of the rotational motion of the molecule. For diatomic gas molecules, the number of degrees of freedom of rotational motion is 2, for three and polyatomic molecules - 3.
Since the distribution of the energy of motion of a molecule over all degrees of freedom is uniform, and the number of molecules in one kilomole of gas is equal to Nμ, the internal energy of one kilomole of an ideal gas can be obtained by multiplying expression (4.11) by the number of molecules in one kilomole and by the number of degrees of freedom of motion of a molecule of a given gas .

where Uμ is the internal energy of a kilomol of gas in J/kmol, i is the number of degrees of freedom of movement of a gas molecule.
For 1 - atomic gas i = 3, for 2 - atomic gas i = 5, for 3 - atomic and polyatomic gases i = 6.
Electricity. Conditions for the existence of electric current. EMF. Ohm's law for a complete circuit. Work and current power. Joule-Lenz law.
Among the conditions necessary for the existence of an electric current there are: the presence of free electric charges in the medium and the creation of an electric field in the medium. An electric field in a medium is necessary to create directional movement of free charges. As is known, a charge q in an electric field of intensity E is acted upon by a force F = qE, which causes free charges to move in the direction of the electric field. A sign of the existence of an electric field in a conductor is the presence of a non-zero potential difference between any two points of the conductor.
However, electrical forces cannot maintain an electric current for a long time. The directed movement of electric charges after some time leads to equalization of potentials at the ends of the conductor and, consequently, to the disappearance of the electric field in it. To maintain current in an electrical circuit, charges must be subject to forces of a non-electrical nature (external forces) in addition to Coulomb forces. A device that creates external forces, maintains a potential difference in a circuit and converts various types of energy into electrical energy is called a current source.
Conditions for the existence of electric current:
presence of free charge carriers
· presence of potential difference. these are the conditions for the occurrence of current. for the current to exist
· closed circuit
· a source of external forces that maintains the potential difference.
Any forces acting on electrically charged particles, with the exception of electrostatic (Coulomb) forces, are called extraneous forces.
Electromotive force.
Electromotive force (EMF) is a scalar physical quantity that characterizes the work of external (non-potential) forces in direct or alternating current sources. In a closed conducting circuit, the EMF is equal to the work of these forces to move a single positive charge along the circuit.
The unit of EMF, like voltage, is the volt. We can talk about electromotive force at any part of the circuit. The electromotive force of a galvanic cell is numerically equal to the work of external forces when moving a single positive charge inside the element from its negative pole to its positive one. The sign of the EMF is determined depending on the arbitrarily chosen direction of bypass of the section of the circuit where the current source is turned on.
Ohm's law for a complete circuit.
Let's consider the simplest complete circuit consisting of a current source and a resistor with resistance R. A current source having an emf ε has a resistance r, it is called the internal resistance of the current source. To obtain Ohm's law for a complete circuit, we use the law of conservation of energy.
Let a charge q pass through the cross section of the conductor during a time Δt. Then, according to the formula, the work done by external forces when moving a charge q is equal to . From the definition of current strength we have: q = IΔt. Hence, .
Due to the work of external forces, when current passes through the circuit, an amount of heat is released on its external and internal sections of the circuit, according to the Joule-Lenz law equal:
According to the law of conservation of energy, A st = Q, therefore Hence Thus, the emf of the current source is equal to the sum of the voltage drops in the external and internal sections of the circuit.