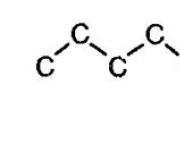
The first 10 alkanes. International nomenclature of alkanes
In chemistry, alkanes are saturated hydrocarbons in which the carbon chain is open and consists of carbon linked to each other by single bonds. Another characteristic feature of alkanes is that they do not contain double or triple bonds at all. Sometimes alkanes are called paraffins; the fact is that paraffins are actually a mixture of saturated carbons, that is, alkanes.
Alkanes formula
The alkane formula can be written as:
In this case, n is greater than or equal to 1.
Alkanes are characterized by isomerism of the carbon skeleton. In this case, the connections can take on different geometric shapes, as for example shown in the picture below.

Isomerism of the carbon skeleton of alkanes
As the carbon chain grows, the number of isomers also increases. For example, butane has two isomers.

Preparation of alkanes
Alkane is usually obtained by various synthetic methods. For example, one of the methods for producing an alkane involves a “hydrogenation” reaction, when alkanes are produced from unsaturated carbohydrates under the influence of a catalyst and at temperature.
Physical properties of alkanes
Alkanes differ from other substances by their complete lack of color, and they are also insoluble in water. The melting point of alkanes increases with increasing molecular weight and hydrocarbon chain length. That is, the more branched an alkane is, the higher its combustion and melting temperature. Gaseous alkanes burn with a pale blue or colorless flame, while releasing a lot of heat.
Chemical properties of alkanes
Alkanes are chemically inactive substances, due to the strength of strong sigma bonds C-C and C-H. In this case, the C-C bonds are non-polar, and the C-H bonds are low-polar. And since all these are low-polarized types of bonds that belong to the sigma type, they will be broken according to a homolytic mechanism, as a result of which radicals are formed. And as a consequence, the chemical properties of alkanes are mainly radical substitution reactions.
This is the formula for radical substitution of alkanes (halogenation of alkanes).
In addition, one can also distinguish such chemical reactions as the nitration of alkanes (Konovalov reaction).
This reaction occurs at a temperature of 140 C, and it is best with a tertiary carbon atom.
Cracking of alkanes - this reaction occurs under the action of high temperatures and catalysts. Then conditions are created when higher alkanes can break their bonds to form alkanes of a lower order.
DEFINITION
Alkanes are called saturated hydrocarbons, the molecules of which consist of carbon and hydrogen atoms connected to each other only by σ bonds.
Under normal conditions (at 25 o C and atmospheric pressure), the first four members of the homologous series of alkanes (C 1 - C 4) are gases. Normal alkanes from pentane to heptadecane (C 5 - C 17) are liquids, starting from C 18 and above are solids. As the relative molecular weight increases, the boiling and melting points of alkanes increase. With the same number of carbon atoms in the molecule, branched alkanes have lower boiling points than normal alkanes. The structure of the alkane molecule using methane as an example is shown in Fig. 1.
Rice. 1. The structure of the methane molecule.
Alkanes are practically insoluble in water, since their molecules are low-polar and do not interact with water molecules. Liquid alkanes mix easily with each other. They dissolve well in non-polar organic solvents such as benzene, carbon tetrachloride, diethyl ether, etc.
Preparation of alkanes
The main sources of various saturated hydrocarbons containing up to 40 carbon atoms are oil and natural gas. Alkanes with a small number of carbon atoms (1 - 10) can be isolated by fractional distillation of natural gas or the gasoline fraction of oil.
There are industrial (I) and laboratory (II) methods for producing alkanes.
C + H 2 → CH 4 (kat = Ni, t 0);
CO + 3H 2 → CH 4 + H 2 O (kat = Ni, t 0 = 200 - 300);
CO 2 + 4H 2 → CH 4 + 2H 2 O (kat, t 0).
— hydrogenation of unsaturated hydrocarbons
CH 3 -CH=CH 2 + H 2 →CH 3 -CH 2 -CH 3 (kat = Ni, t 0);
- reduction of haloalkanes
C 2 H 5 I + HI →C 2 H 6 + I 2 (t 0);
- alkaline melting reactions of salts of monobasic organic acids
C 2 H 5 -COONa + NaOH → C 2 H 6 + Na 2 CO 3 (t 0);
— interaction of haloalkanes with sodium metal (Wurtz reaction)
2C 2 H 5 Br + 2Na → CH 3 -CH 2 -CH 2 -CH 3 + 2NaBr;
— electrolysis of salts of monobasic organic acids
2C 2 H 5 COONa + 2H 2 O → H 2 + 2NaOH + C 4 H 10 + 2CO 2 ;
K(-): 2H 2 O + 2e → H 2 + 2OH - ;
A(+):2C 2 H 5 COO — -2e → 2C 2 H 5 COO + → 2C 2 H 5 + + 2CO 2 .
Chemical properties of alkanes
Alkanes are among the least reactive organic compounds, which is explained by their structure.
Alkanes under normal conditions do not react with concentrated acids, molten and concentrated alkalis, alkali metals, halogens (except fluorine), potassium permanganate and potassium dichromate in an acidic environment.
For alkanes, the most typical reactions are those that proceed by a radical mechanism. Homolytic cleavage of C-H and C-C bonds is energetically more favorable than their heterolytic cleavage.
Radical substitution reactions most easily occur at the tertiary carbon atom, then at the secondary carbon atom, and lastly at the primary carbon atom.
All chemical transformations of alkanes proceed with splitting:
1) C-H bonds
— halogenation (S R)
CH 4 + Cl 2 → CH 3 Cl + HCl ( hv);
CH 3 -CH 2 -CH 3 + Br 2 → CH 3 -CHBr-CH 3 + HBr ( hv).
- nitration (S R)
CH 3 -C(CH 3)H-CH 3 + HONO 2 (dilute) → CH 3 -C(NO 2)H-CH 3 + H 2 O (t 0).
— sulfochlorination (S R)
R-H + SO 2 + Cl 2 → RSO 2 Cl + HCl ( hv).
- dehydrogenation
CH 3 -CH 3 → CH 2 =CH 2 + H 2 (kat = Ni, t 0).
- dehydrocyclization
CH 3 (CH 2) 4 CH 3 → C 6 H 6 + 4H 2 (kat = Cr 2 O 3, t 0).
2) C-H and C-C bonds
- isomerization (intramolecular rearrangement)
CH 3 -CH 2 -CH 2 -CH 3 →CH 3 -C(CH 3)H-CH 3 (kat=AlCl 3, t 0).
- oxidation
2CH 3 -CH 2 -CH 2 -CH 3 + 5O 2 → 4CH 3 COOH + 2H 2 O (t 0 , p);
C n H 2n+2 + (1.5n + 0.5) O 2 → nCO 2 + (n+1) H 2 O (t 0).
Applications of alkanes
Alkanes have found application in various industries. Let us consider in more detail, using the example of some representatives of the homologous series, as well as mixtures of alkanes.
Methane forms the raw material basis for the most important chemical industrial processes for the production of carbon and hydrogen, acetylene, oxygen-containing organic compounds - alcohols, aldehydes, acids. Propane is used as automobile fuel. Butane is used to produce butadiene, which is a raw material for the production of synthetic rubber.
A mixture of liquid and solid alkanes up to C 25, called Vaseline, is used in medicine as the basis of ointments. A mixture of solid alkanes C 18 - C 25 (paraffin) is used to impregnate various materials (paper, fabrics, wood) to give them hydrophobic properties, i.e. non-wetting with water. In medicine it is used for physiotherapeutic procedures (paraffin treatment).
Examples of problem solving
EXAMPLE 1
| Exercise | When chlorinating methane, 1.54 g of a compound was obtained, the vapor density of which in air is 5.31. Calculate the mass of manganese dioxide MnO 2 that will be required to produce chlorine if the ratio of the volumes of methane and chlorine introduced into the reaction is 1:2. |
| Solution | The ratio of the mass of a given gas to the mass of another gas taken in the same volume, at the same temperature and the same pressure is called the relative density of the first gas to the second. This value shows how many times the first gas is heavier or lighter than the second gas. The relative molecular weight of air is taken to be 29 (taking into account the content of nitrogen, oxygen and other gases in the air). It should be noted that the concept of “relative molecular mass of air” is used conditionally, since air is a mixture of gases. Let's find the molar mass of the gas formed during the chlorination of methane: M gas = 29 ×D air (gas) = 29 × 5.31 = 154 g/mol. This is carbon tetrachloride - CCl 4. Let's write the reaction equation and arrange the stoichiometric coefficients: CH 4 + 4Cl 2 = CCl 4 + 4HCl. Let's calculate the amount of carbon tetrachloride substance: n(CCl 4) = m(CCl 4) / M(CCl 4); n(CCl 4) = 1.54 / 154 = 0.01 mol. According to the reaction equation n(CCl 4) : n(CH 4) = 1: 1, which means n(CH 4) = n(CCl 4) = 0.01 mol. Then, the amount of chlorine substance should be equal to n(Cl 2) = 2 × 4 n(CH 4), i.e. n(Cl 2) = 8 × 0.01 = 0.08 mol. Let us write the reaction equation for the production of chlorine: MnO 2 + 4HCl = MnCl 2 + Cl 2 + 2H 2 O. The number of moles of manganese dioxide is 0.08 mol, because n(Cl 2) : n(MnO 2) = 1: 1. Find the mass of manganese dioxide: m(MnO 2) = n(MnO 2) × M(MnO 2); M(MnO 2) = Ar(Mn) + 2×Ar(O) = 55 + 2×16 = 87 g/mol; m(MnO 2) = 0.08 × 87 = 10.4 g. |
| Answer | The mass of manganese dioxide is 10.4 g. |
EXAMPLE 2
| Exercise | Determine the molecular formula of trichloroalkane, the mass fraction of chlorine in which is 72.20%. Draw up the structural formulas of all possible isomers and give the names of the substances according to the IUPAC substitutive nomenclature. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Answer | Let's write the general formula of trichloroalkean: C n H 2 n -1 Cl 3 . According to the formula ω(Cl) = 3×Ar(Cl) / Mr(C n H 2 n -1 Cl 3) × 100% Let's calculate the molecular weight of trichloroalkane: Mr(C n H 2 n -1 Cl 3) = 3 × 35.5 / 72.20 × 100% = 147.5. Let's find the value of n: 12n + 2n - 1 + 35.5×3 = 147.5; Therefore, the formula of trichloroalkane is C 3 H 5 Cl 3. Let's compose the structural formulas of the isomers: 1,2,3-trichloropropane (1), 1,1,2-trichloropropane (2), 1,1,3-trichloropropane (3), 1,1,1-trichloropropane (4) and 1 ,2,2-trichloropropane (5). CH 2 Cl-CHCl-CH 2 Cl (1); CHCl 2 -CHCl-CH 3 (2); CHCl 2 -CH 2 -CH 2 Cl (3); CCl 3 -CH 2 -CH 3 (4); alkane or paraffin(historical name, which also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane is made up of hydrogen and carbon atoms arranged in a tree-like structure in which all carbon-carbon bonds are single.Alkanes have a general chemical formula C n H 2n + 2. Alkanes range in complexity from the simplest case of methane, CH 4, where n = 1 (sometimes called the original molecule), to arbitrarily large molecules. Chemical structure of methane, the simplest alkaneApart from this standard definition called by the International Union of Pure and Applied Chemistry, in the use of some authors the term alkane applies to any saturated hydrocarbon, including those that are either monocyclic (i.e. cycloalkanes) or polycyclic. In an alkane, each carbon atom has 4 bonds (either C-C or C-H), and each hydrogen atom is attached to one of the carbon atoms (as in a C-H bond). The longest series of bonded carbon atoms in a molecule is known as its carbon skeleton or carbon backbone. The number of carbon atoms can be thought of as the size of the alkane. One group of higher alkanes are waxes, solids at standard ambient temperature and pressure (STAP), for which the number of carbon atoms in the carbon chain is about 17 times greater. With repeated -CH 2 — alkanes constitute a homologous series of organic compounds in which the groups differ in molecular weight by a multiple of 14.03 μm (the total mass of each such methylene unit, which contains a single carbon atom with a mass of 12.01 μm and two hydrogen atoms with a mass of ~ 1.01 μm every). Alkanes are not very reactive and have little biological activity. They can be thought of as molecular trees on which the more active/reactive functional groups of biological molecules can be suspended. Alkanes have two main sources: petroleum (crude oil) and natural gas. An alkyl group, usually abbreviated as R, is a functional group that, like an alkane, consists solely of bonded acyclic carbon and hydrogen atoms, such as a methyl or ethyl group. Classification structure
Saturated hydrocarbons are hydrocarbons that have only single covalent bonds between their carbon atoms. They may represent:
Isobutane for 2-methylpropane Chemical properties of alkanes- you can study this, in a complete, understandable presentation. Physical properties of alkanesAll alkanes are colorless and odorless. Table of alkanes.
Boiling point Alkanes experience intermolecular van der Waals forces. Stronger intermolecular van der Waals forces cause higher boiling points of alkanes. There are two determinants for the strength of Van Der Waals forces:
Under standard conditions from CH 4 to C 4 H 10, alkanes are gaseous; From C5H12 to C17H36 they are liquids; And after C 18 H 38 they are solid. Since the boiling points of alkanes are primarily determined by weight, it should not be surprising that the boiling point has an almost linear relationship with the size (molecular weight) of the molecule. Typically, the boiling point increases by 20-30 °C for each carbon added to the chain. This rule also applies to other homologous series.
A straight-chain alkane will have a higher boiling point than a branched-chain alkane due to the greater surface area in contact, thus greater van der Waals forces between adjacent molecules. For example, compare isobutane (2-methylpropane) and n-butane (butane), which boil at -12 and 0 °C, and 2,2-dimethylbutane and 2,3-dimethylbutane, which boil at 50 and 58 °C, respectively . In the latter case, two molecules of 2,3-dimethylbutane can "click" together better than the cross-shaped 2,2-dimethylbutane, hence the large van der Waals forces On the other hand, cycloalkanes tend to have higher boiling points than their linear counterparts due to the locked conformations of the molecules, which provide a plane of intermolecular contact. Melting points The melting points of alkanes have a similar trend to their boiling points for the same reason as above. That is, (other things being equal) the larger the molecule, the higher the melting point. There is one significant difference between boiling points and melting points. Solids have a more rigid and fixed structure than liquids. This rigid structure requires energy to break down. Thus, for better bonding of solid structures, more energy will be required to break. For alkanes, this can be seen in the graph above (i.e. the green line). Odd-numbered alkanes have a lower tendency to melt than even-numbered alkanes. This is explained by the fact that even numbered alkanes pack well in the solid phase, forming a well-organized structure that requires more energy to break. Odd-numbered alkanes pack less well, and therefore an organized compact structure with a looser one requires less energy to break. The melting points of branched-chain alkanes can be either higher or lower than the corresponding straight-chain alkanes, again depending on the ability of the alkane in question to stack well in the solid phase: this is especially true for isoalkanes (2-methyl isomers), which often have melting temperatures higher than those of their linear analogues. Conductivity and solubility Alkanes do not conduct electricity and are not polarized by an electric field. For this reason, they do not form hydrogen bonds and are insoluble in polar solvents such as water. Because the hydrogen bonds between individual water molecules are aligned away from the alkane molecule, the coexistence of the alkane and water results in increased molecular order (decreased entropy). Since there is no significant cohesion between water molecules and alkane molecules, the second law of thermodynamics suggests that this decrease in entropy should be minimized by minimizing contact between the alkane and water: alkanes are said to be hydrophobic in the sense that they repel water. Their solubility in non-polar solvents is relatively good, a property called lipophilicity. Various alkanes, for example, are mixed in all proportions with each other. The density of alkanes generally increases with the number of carbon atoms, but remains less than that of water. Therefore, the alkanes form the top layer as an alkane-water mixture. Molecular geometry
The molecular structure of alkanes directly affects their physical and chemical characteristics. It is derived from the electron configuration of carbon, which has four valence electrons. The carbon atoms in alkanes are always sp 3 hybridized, that is, the valence electrons are said to be in four equivalent orbitals, derived from a combination of 2 s orbitals and three 2p orbitals. These orbitals, having the same energies, are spatially arranged in the form of a tetrahedron, the angle between them cos -1 (- 1/3) ≈ 109.47 °. Bond lengths and bond angles An alkane molecule has only C-H and C-C single bonds. The former are a consequence of the overlap of the sp 3 orbital of carbon with the 1s orbital of hydrogen; The latter is due to the overlap of two sp 3 orbitals on different carbon atoms. The bond lengths are 1.09 × 10 -10 m for the C-H bond and 1.54 × 10 -10 μm for the C-C bond. The spatial arrangement of the bonds is similar to that of the four sp3 orbitals—they are arranged tetrahedrally with an angle of 109.47° between them. Structural formulas that present bonds as being at right angles to each other, while general and useful, are not true. Conformation The structural formula and bond angles are usually insufficient to fully describe the geometry of a molecule. There is one more degree of freedom for each carbon-carbon bond: the torsion angle between the atoms or groups bonded to the atoms at each end of the bond. The spatial arrangement described by the angles of torsion of a molecule is known as its shape. Ethane forms the simplest case for studying the conformation of alkanes, since there is only one C-C bond. If you look down the C-C bond axis, you will see what is called the Newman projection. The hydrogen atoms on both the front and back carbon atoms have an angle of 120° between them, which is due to the projection of the base of the tetrahedron onto a flat plane. However, the angle of torsion between a given hydrogen atom attached to the front carbon and a given hydrogen atom attached to the back carbon can be freely varied from 0° to 360°. This is a consequence of free rotation around a simple carbon-carbon bond. Despite this apparent freedom, only two extreme conformations are important: the eclipsing conformation and the step conformation.  Ball and twin screw models of two ethane rotamers Ball and twin screw models of two ethane rotamers The two conformations, also known as rotamers, differ in energy: the staggered conformation is 12.6 kJ/mol lower in energy (more stable) than the eclipsed conformation (least stable). This difference in energy between the two conformations, called torsional energy, is small compared to the thermal energy of an ethane molecule at ambient temperature. Constant rotation around the C-C bond. The time required for the transition of an ethane molecule from one staggered conformation to another, which is equivalent to the rotation of one CH3 group by 120 ° relative to the other, is on the order of 10 -11 s.  Projections of two conformations of ethane: eclipsed on the left side, checkerboard on the right. Projections of two conformations of ethane: eclipsed on the left side, checkerboard on the right. Higher alkanes are more complex, but based on similar principles, with the antiperiplanar conformation always favored around each carbon-carbon bond. For this reason, alkanes are usually shown in a zigzag pattern in diagrams and models. The actual structure will always differ somewhat from these idealized forms, since the differences in energy between conformations are small compared to the thermal energy of the molecules, since alkane molecules do not have a fixed structural form, no matter what the model may show. Spectroscopic properties Almost all organic compounds contain carbon-carbon and carbon-hydrogen bonds and therefore show some of the features of alkanes in their spectra. Alkanes are distinguished by the absence of other groups and, therefore, the absence of other characteristic spectroscopic features of various functional groups, such as -OH, -CHO, -COOH, etc. Infrared spectroscopy The carbon-hydrogen stretching mode gives strong absorption between 2850 and 2960 cm -1, while the carbon-carbon stretching mode absorbs from 800 to 1300 cm -1. Carbon-hydrogen bending methods depend on the nature of the group: methyl groups show bands at 1450 cm -1 and 1375 cm -1 , while methylene groups show bands at 1465 cm -1 and 1450 cm -1 . Carbon chains with more than four carbon atoms show weak absorption at about 725 cm -1. NMR spectroscopy Proton resonances of alkanes are usually found at δH = 0.5-1.5. Resonances of carbon 13 depend on the number of hydrogen atoms bonded to carbon: δ C = 8-30 (primary, methyl, -CH 3), 15-55 (secondary, methylene, -CH 2 -), 20-60 (tertiary, Metin , C-H) and quaternary. Carbon-13 resonance of quaternary carbon atoms is characterized by weakness due to the absence of the nuclear Overhauser effect and long relaxation times, and can be missed in weak samples or samples that have not been processed for a sufficiently long time. Mass spectrometryAlkanes have high ionization energy, while molecular ions usually have weak ionization energies. Fragmentation fragmentation can be difficult to interpret, but in the case of branched alkanes, the carbon chain is preferentially cleaved at tertiary or quaternary carbons due to the relative stability of the resulting free radicals. The fragment resulting from the loss of one methyl group (M-15) is often missing, and the other fragment is often separated by intervals of fourteen mass units, corresponding to the sequential loss of CH 2 groups. Methods for producing alkanesYou can also learn and study about methods for obtaining alkanes in this article. DEFINITION Alkanes– saturated (aliphatic) hydrocarbons, the composition of which is expressed by the formula C n H 2 n +2. Alkanes form a homologous series, each chemical compound of which differs in composition from the next and previous ones by the same number of carbon and hydrogen atoms - CH 2, and the substances included in the homologous series are called homologues. The homologous series of alkanes is presented in Table 1. Table 1. Homologous series of alkanes. In alkane molecules, primary (i.e. connected by one bond), secondary (i.e. connected by two bonds), tertiary (i.e. connected by three bonds) and quaternary (i.e. connected by four bonds) carbon atoms are distinguished. C 1 H3 – C 2 H 2 – C 1 H 3 (1 – primary, 2 – secondary carbon atoms) CH 3 –C 3 H(CH 3) – CH 3 (3-tertiary carbon atom) CH 3 – C 4 (CH 3) 3 – CH 3 (4-quaternary carbon atom) Alkanes are characterized by structural isomerism (carbon skeleton isomerism). Thus, pentane has the following isomers: CH 3 -CH 2 -CH 2 -CH 2 -CH 3 (pentane) CH 3 –CH(CH 3)-CH 2 -CH 3 (2-methylbutane) CH 3 -C(CH 3) 2 -CH 3 (2,2 – dimethylpropane) Alkanes, starting with heptane, are characterized by optical isomerism. The carbon atoms in saturated hydrocarbons are in sp 3 hybridization. The angles between bonds in alkane molecules are 109.5. Chemical properties of alkanesUnder normal conditions, alkanes are chemically inert - they do not react with either acids or alkalis. This is explained by the high strength of C-C and C-H bonds. Non-polar C-C and C-H bonds can only be cleaved homolytically under the influence of active free radicals. Therefore, alkanes enter into reactions that proceed by the radical substitution mechanism. In radical reactions, hydrogen atoms are first replaced at tertiary carbon atoms, then at secondary and primary carbon atoms. Radical substitution reactions have a chain nature. The main stages: nucleation (initiation) of the chain (1) - occurs under the influence of UV radiation and leads to the formation of free radicals, chain growth (2) - occurs due to the abstraction of a hydrogen atom from the alkane molecule; chain termination (3) – occurs when two identical or different radicals collide. X:X → 2X . (1) R:H+X . → HX + R . (2) R . + X:X → R:X + X . (2) R . + R . → R:R (3) R . +X . → R:X (3) X . +X . → X:X (3) Halogenation. When alkanes interact with chlorine and bromine under the action of UV radiation or high temperature, a mixture of products from mono- to polyhalogen-substituted alkanes is formed: CH 3 Cl +Cl 2 = CH 2 Cl 2 + HCl (dichloromethane) CH 2 Cl 2 + Cl 2 = CHCl 3 + HCl (trichloromethane) CHCl 3 +Cl 2 = CCl 4 + HCl (carbon tetrachloride) Nitration (Konovalov reaction). When dilute nitric acid acts on alkanes at 140C and low pressure, a radical reaction occurs: CH 3 -CH 3 +HNO 3 = CH 3 -CH 2 -NO 2 (nitroethane) + H 2 O Sulfochlorination and sulfoxidation. Direct sulfonation of alkanes is difficult and is most often accompanied by oxidation, resulting in the formation of alkanesulfonyl chlorides: R-H + SO 2 + Cl 2 → R-SO 3 Cl + HCl The sulfonic oxidation reaction proceeds similarly, only in this case alkanesulfonic acids are formed: R-H + SO 2 + ½ O 2 → R-SO 3 H Cracking– radical cleavage of C-C bonds. Occurs when heated and in the presence of catalysts. When higher alkanes are cracked, alkenes are formed; when methane and ethane are cracked, acetylene is formed: C 8 H 18 = C 4 H 10 (butane) + C 3 H 8 (propane) 2CH 4 = C 2 H 2 (acetylene) + 3H 2 Oxidation. The mild oxidation of methane with atmospheric oxygen can produce methanol, formic aldehyde or formic acid. In air, alkanes burn to carbon dioxide and water: C n H 2 n +2 + (3n+1)/2 O 2 = nCO 2 + (n+1)H 2 O Physical properties of alkanesUnder normal conditions, C 1 -C 4 are gases, C 5 -C 17 are liquids, and starting from C 18 are solids. Alkanes are practically insoluble in water, but are highly soluble in non-polar solvents, such as benzene. Thus, methane CH 4 (swamp, mine gas) is a colorless and odorless gas, highly soluble in ethanol, ether, hydrocarbons, but poorly soluble in water. Methane is used as a high-calorie fuel in natural gas, as a raw material for the production of hydrogen, acetylene, chloroform and other organic substances on an industrial scale. Propane C 3 H 8 and butane C 4 H 10 are gases used in everyday life as bottled gases due to their easy liquefaction. Propane is used as a car fuel because it is more environmentally friendly than gasoline. Butane is the raw material for the production of 1,3-butadiene, which is used in the production of synthetic rubber. Preparation of alkanesAlkanes are obtained from natural sources - natural gas (80-90% - methane, 2-3% - ethane and other saturated hydrocarbons), coal, peat, wood, oil and rock wax. There are laboratory and industrial methods for producing alkanes. In industry, alkanes are obtained from bituminous coal (1) or by the Fischer-Tropsch reaction (2): nC + (n+1)H 2 = C n H 2 n +2 (1) nCO + (2n+1)H 2 = C n H 2 n +2 + H 2 O (2) Laboratory methods for producing alkanes include: hydrogenation of unsaturated hydrocarbons by heating and in the presence of catalysts (Ni, Pt, Pd) (1), the interaction of water with organometallic compounds (2), electrolysis of carboxylic acids (3), by decarboxylation reactions (4) and Wurtz (5) and in other ways. R 1 -C≡C-R 2 (alkyne) → R 1 -CH = CH-R 2 (alkene) → R 1 -CH 2 – CH 2 -R 2 (alkane) (1) R-Cl + Mg → R-Mg-Cl + H 2 O → R-H (alkane) + Mg(OH)Cl (2) CH 3 COONa↔ CH 3 COO — + Na + 2CH 3 COO - → 2CO 2 + C 2 H 6 (ethane) (3) CH 3 COONa + NaOH → CH 4 + Na 2 CO 3 (4) R 1 -Cl +2Na +Cl-R 2 →2NaCl + R 1 -R 2 (5) Examples of problem solvingEXAMPLE 1
Alkanes or aliphatic saturated hydrocarbons are compounds with an open (non-cyclic) chain, in the molecules of which the carbon atoms are connected to each other by a σ bond. The carbon atom in alkanes is in a state of sp 3 hybridization. Alkanes form a homologous series in which each member differs by a constant structural unit -CH 2 -, which is called a homological difference. The simplest representative is methane CH4.
IUPAC nomenclature Prefixes are used in the names of alkanes n-, second-, iso, tert-, neo:
The nomenclature of branched alkanes is based on the following basic rules:
It should be noted that for complex residues (groups) multiplying prefixes like bis-, tris-, tetrakis- other.
Industrial extraction methods 1. Extraction of alkanes gas. Natural gas consists mainly of methane and small admixtures of ethane, propane, and butane. Gas under pressure at low temperatures is divided into appropriate fractions. 2. Extraction of alkanes from oil. Crude oil is purified and processed (distillation, fractionation, cracking). Mixtures or individual compounds are obtained from processed products. 3. Hydrogenation of coal (method of F. Bergius, 1925). Hard or brown coal in autoclaves at 30 MPa in the presence of catalysts (oxides and sulfides of Fe, Mo, W, Ni) in a hydrocarbon environment is hydrogenated and converted into alkanes, the so-called motor fuel: nC + (n+1)H 2 = C n H 2n+2 4. Oxosynthesis of alkanes (method of F. Fischer - G. Tropsch, 1922). Using the Fischer-Tropsch method, alkanes are obtained from synthesis gas. Synthesis gas is a mixture of CO and H 2 with different ratios. It is obtained from methane by one of the reactions that occur at 800-900°C in the presence of nickel oxide NiO supported on Al 2 O 3: CH 4 + H 2 O ⇄ CO + 3H 2 CH 4 + CO 2 ⇄ 2CO + 2H 2 2CH 4 + O 2 ⇄ 2CO + 4H 2 Alkanes are obtained by the reaction (temperature about 300°C, Fe-Co catalyst): nCO + (2n+1)H 2 → C n H 2n+2 + nH 2 O The resulting mixture of hydrocarbons, consisting mainly of alkanes of the structure (n = 12-18), is called “syntin”. 5. Dry distillation. Alkanes are obtained in relatively small quantities by dry distillation or heating of coal, shale, wood, and peat without access to air. The approximate composition of the resulting mixture is 60% hydrogen, 25% methane and 3-5% ethylene. Laboratory extraction methods 1. Preparation from haloalkyls 1.1. Reaction with metallic sodium (Wurz, 1855). The reaction consists of the interaction of an alkali metal with a haloalkyl and is used for the synthesis of higher symmetrical alkanes: 2CH 3 -I + 2Na ⇄ CH 3 -CH 3 + 2NaI If two different haloalkyls participate in the reaction, a mixture of alkanes is formed: 3CH 3 -I + 3CH 3 CH 2 -I + 6Na → CH 3 -CH 3 + CH 3 CH 2 CH 3 + CH 3 CH 2 CH 2 CH 3 + 6NaI 1.2 Interaction with lithium dialkyl cuprates. The method (sometimes called the E. Core - H. House reaction) involves the interaction of reactive lithium dialkyl cuprates R 2 CuLi with haloalkyls. First, lithium metal reacts with a haloalkane in an ether environment. Next, the corresponding alkyl lithium reacts with copper(I) halide to form a soluble lithium dialkyl cuprate: CH 3 Cl + 2Li → CH 3 Li + LiCl 2CH 3 Li + CuI → (CH 3 ) 2 CuLi + LiI When such a lithium dialkyl cuprate reacts with the corresponding haloalkyl, the final compound is formed: (CH 3 ) 2 CuLi + 2CH 3 (CH 2 ) 6 CH 2 -I → 2CH 3 (CH 2 ) 6 CH 2 -CH 3 + LiI + CuI The method makes it possible to achieve a yield of alkanes of almost 100% when using primary haloalkyls. With their secondary or tertiary structure, the yield is 30-55%. The nature of the alkyl component in lithium dialkyl cuprate has little effect on the yield of the alkane. 1.3 Reduction of haloalkyls. It is possible to reduce haloalkyls with catalytically excited molecular hydrogen, atomic hydrogen, iodine, etc.: CH 3 I + H 2 → CH 4 + HI (Pd catalyst) CH 3 CH 2 I + 2H → CH 3 CH 3 + HI CH 3 I + HI → CH 4 + I 2 The method has no preparative value; a strong reducing agent is often used - iodine. 2. Preparation from salts of carboxylic acids. R-COONa ⇄ R-COO - + Na + At the anode, the carboxylic acid anion is oxidized, forming a free radical, and is easily decarboxylated or eliminated by CO 2 . Alkyl radicals are further converted into alkanes due to recombination: R-COO - → R-COO . + e - R-COO. →R. +CO2 R. +R. → R-R Kolbe's preparative method is considered effective in the presence of the corresponding carboxylic acids and the impossibility of using other synthesis methods. 2.2 Fusion of salts of carboxylic acids with alkali. Alkali metal salts of carboxylic acids, when combined with alkali, form alkanes: CH 3 CH 2 COONa + NaOH → Na 2 CO 3 + CH 3 CH 3 3. Reduction of oxygen-containing compounds(alcohols, ketones, carboxylic acids) . The reducing agents are the above-mentioned compounds. Most often, iodine is used, which is capable of reducing even ketones: The first four representatives of alkanes from methane to butane (C 1 -C 4) are gases, from pentane to pentadecane (C 5 -C 15 - liquids, from hexadecane (C 16) - solids substances. An increase in their molecular weights leads to an increase in boiling and melting points, whereby alkanes with a branched chain boil at a lower temperature than alkanes of a normal structure. This is explained by the lower van der Waals interaction between the molecules of branched hydrocarbons in the liquid state. The melting point of even homologs is higher in compared with the temperature, respectively, for odd ones. Alkanes are much lighter than water, non-polar and difficult to polarize, but they are soluble in most non-polar solvents, due to which they themselves can be a solvent for many organic compounds. Categories
|