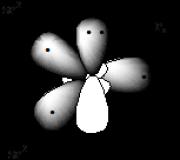
Electronic structure of the pyrrole nitrogen atom. Nitrogen compounds
Nitrogen in natureIn the air
1%
21%
nitrogen
oxygen
carbon dioxide,
inert gases
78%
04.02.2018
Kartashova L.A.
Nitrogen cycle in nature
04.02.2018Kartashova L.A.
Properties of nitrogen
In the free state, nitrogen exists inin the form of diatomic N2 molecules. In these
molecules, two nitrogen atoms are very bonded
strong triple covalent bond.
N N
N N
Nitrogen is a colorless, odorless and tasteless gas. Badly
dissolves in water. In liquid state (temp.
boiling point −195.8 °C) – colorless, mobile, like
water, liquid. Density of liquid nitrogen 808
kg/m³. At −209.86 °C nitrogen turns into solid
state in the form of a snow-like mass or
large snow-white crystals.
04.02.2018
Kartashova L.A.
Properties of nitrogen
Under normal conditions, nitrogen reacts only withlithium, forming lithium nitride:
6Li+ N2 = 2Li3N
It reacts with other metals only when heated.
At high temperatures, pressure and in the presence
catalyst, nitrogen reacts with hydrogen to form ammonia:
N2 + 3H2 = 2NH3
At the temperature of the electric arc, it connects to
oxygen, forming nitric oxide (II):
N2 + O2 = 2NO - Q
04.02.2018
Kartashova L.A.
Nitrogen oxides
Non-salt-formingoxide - "laughing gas"
Colorless non-flammable
gas with pleasant
sweetish smell and
taste.
Non-salt-forming
oxide, colorless gas,
poorly soluble in
water. Does not liquefy well;
in liquid and solid
the form has a blue color.
acid oxide,
colorless gas (at zero)
in solid form, bluish in color.
Stable only when
temperatures below -4 °C
Oxide
nitrogen(I)
Oxide
nitrogen(II)
Oxide
nitrogen(III)
acid oxide,
"fox tail" brown,
very poisonous gas
Oxide
nitrogen(IV)
04.02.2018
Acidic oxide.
Colorless, very
flying crystals.
Extremely unstable.
Oxide
nitrogen(V)
Kartashova L.A.
Ammonia
NH
H
H
Ammonia is a colorless gas with a pungent odor.
almost twice lighter than air. Ammonia
you cannot inhale for a long time,
because he is poisonous. Ammonia is very good
dissolves in water.
In the ammonia molecule NH3 there are three covalent
polar bonds between a nitrogen atom and
hydrogen atoms.
H N H
H
04.02.2018
Kartashova L.A.
or
H N H
H
Ammonia production in industry
04.02.2018Kartashova L.A.
10. Obtaining ammonia in the laboratory
04.02.2018Kartashova L.A.
11. Use of ammonia in the national economy
04.02.2018Kartashova L.A.
12. Nitric acid
Nitric acid - colorless, fumingliquid in air, temperature
melting −41.59 °C, boiling +82.6 °C
with partial decomposition.
Solubility of nitric acid in water
not limited.
H O N
04.02.2018
Kartashova L.A.
O
O
13. Chemical properties of nitric acid
Typical properties:a) with basic and amphoteric oxides:
CuO + 2HNO3 = Cu(NO3)2 + H2O
ZnO + 2HNO3 = Cu(NO3)2 + H2O
b) with reasons:
KOH + HNO3 = KNO3+H2O
c) displaces weak acids from their salts:
CaCO3 + 2HNO3 = Ca(NO3)2 + H2O + CO2
When boiling or exposed to light, nitric acid
partially decomposes:
4HNO3 = 2H2O + 4NO2 + O2
04.02.2018
Kartashova L.A.
14. Chemical properties of nitric acid
1. With metals up to N1. With metals up to N
3Zn+8HNO3=3Zn(NO3)2+4H2O+2NO Zn+4HNO3=Zn(NO3)2+2H2O+2NO
2. With metals after H
2. With metals after H
3Cu+8HNO3=3Cu(NO3)2+4H2O+2NO Cu+4HNO3=Cu(NO3)2+2H2O+2NO2
3. With non-metals
S+2HNO3= H2SO4+2NO
3. With non-metals
S+6HNO3= H2SO4+6NO2+2H2O
4. With organic substances
C2H6+HNO3=C2H5NO2
4. Passivates iron, aluminum,
chromium
04.02.2018
Kartashova L.A.
15. Nitric acid salts
Saltsnitrogen
acids
Sodium nitrate
Calcium nitrate
Potassium nitrate
04.02.2018
Ammonium nitrate
Kartashova L.A.
16. Fill in the missing words
In the periodic table D.I. Mendeleev's nitrogenlocated in period 2, group V, main
subgroup. Its serial number is 7, relative
atomic mass 14.
In compounds, nitrogen exhibits oxidation states
+5, +4, +3, +2, +1, -3. The number of protons in a nitrogen atom is 7,
electrons 7, neutrons 7, nuclear charge +7,
electronic formula 1s22s22p3 Formula of higher
oxide N2O5, its character is acidic, formula
higher hydroxide НNO3, volatile formula
hydrogen compound NH3.
04.02.2018
Kartashova L.A.
17. Distribute nitrogen compounds into classes of inorganic compounds
Oxideswrong
N.H.
Acids
wrong
NO
Salts
wrong
NO
wrong
right
right
wrong
NaNO
right
HNO
wrong
N.H.
right
wrong
N2O5
right
Al(NO
2)3
right
NO
wrong)
Fe(NO
3 2
right
LiNO
3
HNO3
3
N2O5
wrong
HNO
2
04.02.2018
2
3
HNO2
3
wrong
NO
2
Kartashova L.A.
2
KNO3
3
3
wrong
NO
2
5
18. Sources of information
Gabrielyan O. S. Chemistry. 9th grade:http://ru.wikipedia.org/wiki
http://dic.academic.ru/dic.nsf/ruwiki/324035
http://www.catalogmineralov.ru/mineral/50.html
http://chemmarket.info/
http://www.alhimikov.net/video/neorganika/menu.html
04.02.2018
Kartashova L.A.
In the pyridine molecule, p,p conjugation takes place. Pyridinic nitrogen, due to its greater electronegativity compared to carbon, shifts the single p-electron density towards itself, generally reducing the electron density of the aromatic ring. Therefore, such systems with pyridine nitrogen are called p-deficient.
When replacing the fragment - CH = CH - with > NH, a five-membered ring appears - pyrrole
1. The pyrrole molecule has a cyclic structure.
2. All carbon atoms in the cycle are in sp 2 hybridization, the nitrogen atom is also sp 2 hybridized, and the nitrogen atom supplies a two-electron P z orbital to a single p-electron cloud.

3. The total π electron density of pyrrole includes 4n+2 = 6 p electrons
In the pyrrole molecule, p,p conjugation takes place. Systems containing pyrrole nitrogen are called p-excess or superaromatic systems. The presence of such a system greatly affects the reactivity of pyrrole.
In natural compounds, the aromatic pyrrole ring is often found in various polynuclear compounds, of which the most important is the porphin nucleus, which is part of hemoglobin and chlorophyll.
A conjugated system of 26 p-electrons (11 double bonds and 2 lone pairs of electrons of pyrrole atoms. The high conjugation energy (840 KJ) indicates the high stability of porphine.
The concept of aromaticity extends not only to neutral molecules, but also to charged ions. _
When replacing the fragment – CH=CH – in benzene with – CH, a carbocyclic – cyclopentadienyl anion arises, which belongs to the non-benzenoid structure. Cyclopentadienyl ion is a component of the drug ferrocene (dicyclopentadienyl iron) and the natural compound azulene.
The cyclopentadienyl anion is formed by the abstraction of a proton from cyclopentadiene-1,3.
Let's consider the aromaticity criteria for the cyclopentadienyl anion:
1) cyclic connection
2) all carbon atoms have sp 2 hybridization
Ferrocene is a sandwich-like organometallic compound (stimulates hematopoiesis and is used for iron deficiency anemia.
The cycloheptatrienyl cation (tropylium cation) is formed from cycloheptatriene-1,3,5 by elimination of the hydride ion.
The tropylium cation is a regular heptagon. An aromatic sextet is formed by the overlap of 6 one-electron and one vacant p z orbital.
Let's consider the aromaticity criteria for tropylium cation:
1) The connection is cyclic
2) All carbon atoms have sp 2 hybridization
3) The general π-electron system includes 4n + 2 = 6 p-electrons
“Structure of the atom and atomic nucleus” - Protons and neutrons. Examples of electronic formulas of atoms. Image of electron orbitals. Calculation of the number of protons, neutrons and electrons. Atom and nucleus. Levels, sublevels and orbitals. Choose the correct answer. Goals. Finding an electron in an atom. Control materials. Write the electronic formula. Opening the core.
“Structure of the nucleus of an atom” - The atom is neutral, because. the charge of the nucleus is equal to the total charge of the electrons. Electrons move around the nucleus. 1919 Rutherford studied the interaction of particles with the nuclei of nitrogen atoms. Foil made of the metal being studied. CONTENTS Module 1 1. Atomic structure. The total number of nucleons in a nucleus is called the mass number and is denoted A.
“Composition of the nucleus of an atom” - The nucleus of an atom of a chemical element. Graph of specific nucleon bonding in a nucleus. Core charge. Scheme of Rutherford's experiments. Dimensions of atomic nuclei. Discovery of the proton. The number of neutrons in the nucleus of an atom. Nuclear forces. Properties of nuclear forces. Proton and neutron. Mass defect. Formula for finding binding energy. Density of nuclear matter.
““Nuclear structure” physics” - How many nucleons do nuclei contain. Helium nucleus. Charge number. New element. The structure of the atomic nucleus. Learn about the history of the discovery of the neutron. Isotopes. A particle that has no charge. Proton-neutron model of the atomic nucleus. Determine the nucleon composition of the nucleus. Neutron. A hero with short arms.
“Composition of the atomic nucleus” - Lesson plan. NUCLEAR FORCES – attractive forces that bind protons and neutrons in the nucleus. Short-range (r = 2.2 * 10-15 m). PROPERTIES They are only forces of attraction. The charge number is equal to the charge of the nucleus, expressed in elementary electrical charges. Does not depend on the presence of charge. Nuclear forces. Mass number.
“Structure of the atomic nucleus” - M - mass number - mass of the nucleus, number of nucleons, number of neutrons M-Z. Radioactivity is proof of the complex structure of atoms. Nuclear chain reaction. Was Prometheus right when he gave people fire? Fission of the atomic nucleus. Geiger counter Wilson chamber. The structure of the atomic nucleus. Repeating and generalizing lesson
Problem 880.
Give examples of nitrogen compounds whose molecules contain bonds formed according to the donor-acceptor mechanism.
Solution:
A bond according to the donor-acceptor mechanism (coordination bond) is formed due to the sharing of an electron pair of one atom (donor) and a vacant orbital of another atom (acceptor). Non-bonding electron pair of nitrogen atom is capable of forming a covalent bond with a hydrogen ion having a free atomic orbital according to the donor-acceptor mechanism. This is how the ammonium cation NH 4 + is formed from an ammonia molecule and a hydrogen ion:
As a result of the formation of a donor-acceptor bond, the non-bonding electron pair of the nitrogen atom becomes a bonding one, and four bonds are formed between one nitrogen atom and four hydrogen atoms:
All four bonds are equivalent in both length and energy.
Such a bond is identical to a covalent bond formed by the usual mechanism, the sharing of unpaired electrons of two atoms.
Ammonia and its derivatives, with the exception of nitrogen trihalides, have a strong electron-donating ability. Therefore, ammonia, as well as almost all compounds having amino groups and groups: are N-donor ligands that form complex compounds with cations of many metals. There are complexes with the following groups:  glycyanate ion:
glycyanate ion: ![]() glycylglycyl cyanate ion: , ethylenediamine:
glycylglycyl cyanate ion: , ethylenediamine:![]() d ethylenetriamine:
d ethylenetriamine:
and others. The connection in complex compounds can be explained by the coordination bond between the non-bonding electron pairs of the nitrogen atom of the ligand and the free orbitals of the complexing agent atom, for example, Cl 2, Cl 2, etc. In ammonia H 3 and amines ![]() as ammonia derivatives. The nitrogen atom can form a coordination bond, for example: ammonium chloride NH 4 Cl, methyl ammonium hydroxide CH 3 -NH 3 -OH, tetramethyl ammonium iodide (CH 3) 4 NI, hydroxide tetraethylammonium(C 2 H 5) 4 NOH, ammonium hydroxide NH 4 OH, phenylamine chloride C6H5NH3+Cl. Some
as ammonia derivatives. The nitrogen atom can form a coordination bond, for example: ammonium chloride NH 4 Cl, methyl ammonium hydroxide CH 3 -NH 3 -OH, tetramethyl ammonium iodide (CH 3) 4 NI, hydroxide tetraethylammonium(C 2 H 5) 4 NOH, ammonium hydroxide NH 4 OH, phenylamine chloride C6H5NH3+Cl. Some
ammonia derivatives, for example: hydrazine: , hydroxylamine: , as well as hydrazonium chloride N 2 H 5 Cl (+1), hydrazonium hydroxide N 2 H 5 (OH) 2 (+2), hydroxylammonium hydroxide OH, hydrazonium hydroxide (+2) N 2 H 6 (OH) 2, hydrazonium chloride (+2) N 2 H 6 Cl 2, hydroxylammonium chloride NH 3 OHCl.
Problem 881.
Describe the electronic structure of the N 2 molecule from the perspective of the BC and MO methods.
Solution:
a) Electronic structure of the N 2 molecule from the standpoint of the valence bond method
The nitrogen atom in the outer electron layer contains two paired electrons in the 2s sublevel and three unpaired electrons in the 2p sublevel, one in each 2p orbital. A covalent bond with three electron pairs is formed between two nitrogen atoms due to the pairing of three unpaired electrons of each atom. The paired electrons of the 2s orbitals of each nitrogen atom do not participate in the formation of bonds. Therefore, the N2 molecule, in accordance with the theory of valence bonds, can be depicted as having non-bonding electron pairs at each nitrogen atom: - = - , but in reality the electron density is concentrated mainly between the atoms. Molecule N 2 has a linear structure. Since the nitrogen atoms in the N molecule 2 are the same, then the dipole moment of the molecule is zero.
b) Electronic structure of the N 2 molecule from the standpoint of the Molecular Orbital method
The electronic structure of the N2 molecule can be explained from the perspective of the molecular orbital method.
From the standpoint of the MO method, the electronic structure of the N2 molecule can be represented as follows:

The molecule has an electronic configuration:
KK(σ)